A History of Single Use Cholangiogram Forceps
WORLD FIRST AUSTRALIAN INNOVATION
A History of Single Use Cholangiogram Forceps
by Michael A. Swan, Founder, Endovision Australia
In late 2006, being aware of TGA’s May 2004 publication “REDUCING PUBLIC HEALTH RISKS
ASSOCIATED WITH REUSABLE MEDICAL DEVICES”, I thought that Flushable Cholangiogram Forceps
might provide the answer.
Tontarra Medizintechnik in Germany proposed several designs until we agreed on the sixth version which
became MAS-006. Later we added a bariatric version MAS-006B. We also assessed a “take-apart”
design but this was discarded due to complexity and relatively small jaw components that we believed
would not withstand the rigorous processing required for reuse.
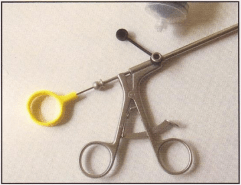

Unbeknown to me at the time Tim Cole, Sterilising Services Manager at Concord and Canterbury
Hospitals in Sydney had been campaigning his CSSD colleagues that cholangiogram forceps could not
be internally inspected and therefore could not be guaranteed clean – “If it is not clean you cannot claim
sterilisation”. Tim had actually taken the bold step of refusing to clean/sterilize cholangiogram forceps and
the Concord Needle was born.
A concerned Tim Cole called me and explained why even a flushable forceps could not be internally
cleaned due to the complex construction of a tube within a tube and the tiny crevices that could harbour
sticky bile and other fluids.
I later proved this fact when I dissected and photographed seven MAS-006. Results were “not bad” to
“awful” but not one instrument was without some sort of debris, either gross or film sticking to the internal
surfaces.
I told Tim that a “take-apart” was not viable and proposed the possibility of “Single Use”. Would hospitals
accept the added cost? That discussion set the wheels in motion and what a journey it has been.
Laparoscopic surgeons however were happy to see an “improved” cholangiogram forceps back in the
market so a compromise decision was made to allow their use until a single use product became
available.
TONTARRA MEDIZINTECHNIK, Tuttlingen, Germany.
Thomas Tontarra, son of the founder, was somewhat cynical about our idea of Single Use – Europe has a
philosophy of reuse. Through my contact Daniel Petrella we convinced T
homas to make a prototype that
was essentially a reusable instrument but with plastic handles to differentiate it from the fully stainless
steel reusable product. This was an important element to minimise confusion in CSSD Departments.
“SINGLE USE ONLY” was to be etched on the shaft.
TRADE MARK
While Tontarra was working on a prototype I spoke with Freehills Trade Mark Attorneys and registered
the trade mark Olsen-Swan. Olsen to recognise Dr. Doug Olsen (of Reddich-Olsen fame) whose name
was attached to the original Olsen Clamp and “Swan” for my own little quest to leave my mark on the
surgical industry.
PATENT
Freehills are also Patent Attorneys. My Trade-Mark enquiries led them to wonder if some sort of patent
protection was possible.
In 2001 the Australian Government introduced the Innovation Patent system. This was designed to assist
small to medium Australian companies by providing a level of patent protection that would encourage
investment in research and development and give the company time to recoup their investment. Instead
of the usual twenty years of a Standard Patent the Innovation Patent has a term of only eight years.
Our Provisional Patent application was lodged on 12th March 2008. Our subsequent Innovation Patent
application was examined by the Patent Office and granted in March 2009.
PRODUCT TO MARKET
Once our Provisional Patent application had been submitted we presented the first MAS-007 to Prof Neil
Merrett of SSWAHS who recommended that it be adopted by hospitals within his area health service –
Bankstown, Liverpool, Campbelltown, Concord, Canterbury and Royal Prince Alfred. The Australian dollar
was worth only about 0.50 Euro at the time so the initial price was $285 ($203 at today’s rates).
Surgeons were delighted with the quality of the new instrument, likening it to that of the reusable product.
CSSD staff were thrilled that they no longer had to worry if they were actually cleaning the reusable.

FURTHER PRODUCT DEVELOPMENT
MAS-007 ORIGINAL was a fully engineered costly to produce instrument. Although our dollar had
strengthened to the point that we could offer MAS-007 at $198 we still needed to drive down production
costs to broaden the acceptance of our clinically desirable innovation.
I hate to think how many hours I spent trying to work out ways to reduce the onerous engineering and
assembly costs. Daniel had sent me some blank handle mouldings and I destroyed several flushable
instruments with hacksaw and file to obtain the shaft assemblies. More hacksaw work on the handles,
some wood carving, glue, a nail to hold the handles together and some very amateurish drawings and I
was ready for a visit to Germany.
Many hours of meeting room, design department and factory time resulted in a design for a mould for the
handle that has remained unchanged (save for a few minor internal variations to facilitate the assembly
process) to this day.

Injection moulds at the time were expensive and Tontarra was committed to other capital intensive
projects so Endovision paid for them. Naturally this cost had to be recovered from the price of the
instrument to you, the customer. Despite the need to recoup tooling and development costs we were able
to reduce the price to $180.
Some “truly left handed” surgeons found our new handle difficult to use so we added a central ratchet
model to the range (MAS-007-R). Making this in small batches was expensive due to labour costs but it
satisfied the need. Bariatric length (MAS-007-B) was added in 2015.
STILL TOO EXPENSIVE
Now that the handle and assembly time issues had been resolved we were still left with the problem that
the jaws, operating rod and Gabel (fork holding the jaws assembly together) were still fully engineered on
expensive multi-head CNC machines that require the full-time attention of a costly technician/operator.
We had to find another way.
MIM (metal injection moulding) or more correctly PIM (powder injection moulding) is an incredible process
which can produce complex metal components at a fraction of the cost of fully engineered parts. German
and British/Indian MIM companies told us that the most costly piece, the Gabel, could not be made by
MIM. Additionally, MIM is a process that can only be viable with higher volume production. More hurdles
to overcome.
In November 2014 I visited the Medica and Compamed (component suppliers to the medical industry)
trade fairs in Düsseldorf where I met with a Chinese company making MIM components and talked about
our needs. They seemed confident that they could make the Gabel.
In May 2015, at a trade fair in Shanghai, the two Directors of Tontarra and I met with representatives of
the MIM company. After much experimentation and redesign over more than a year to accommodate the
special needs of MIM we have achieved our goal of all tip components being made by this process.
MORE COST SAVINGS
In 2016 we moved assembly of the jaw components to the precision German made tubes was moved
from Wurmlingen to a Tontarra affiliate in Hungary. Although this move required the purchase of new
LASER welding and other equipment we will benefit from Hungary’s lower labour costs into the future.
Final assembly and quality control is still performed at Tontarra’s facility in Wurmlingen and the finished
product is shipped to nearby Stuttgart for (our new and improved) packaging and sterilising.
In November 2016 we reduced the price significantly to reflect these additional cost savings.
GUARANTEED SUPPLY
During the transition from the fully engineered product the market was demanding more and more forceps
to a point that we had a short period where we were embarrassed for stock.
Fortunately I had made contact with another Chinese company during my visit to Shanghai in May 2015
and we had begun trialing their product in
selected Melbourne hospitals. We were able to draw on our
stocks of this product to cover the shortfall of MAS-007.
Since that time we have been building stock levels in our Melbourne warehouse to cover at least three
months sales at any given time.
I AM VERY PROUD OF OUR ACHIEVEMENTS IN PATIENT SAFETY
We have changed the way hospitals think about infection control and patient safety in Laparoscopic
Cholecystectomy (keyhole surgery removal of the gall bladder). There are still hospitals who continue to
re-use cholangiogram forceps, quite possibly due to cost. Our much lower priced German instrument and
the addition of an economical Chinese product to our range makes single use much more affordable for
all.
I believe that we have also achieved the goals of the Australian Government when it introduced the
Innovation Patent System in 2001.

With thanks for your continuing support,
Michael Swan

